
Product Details
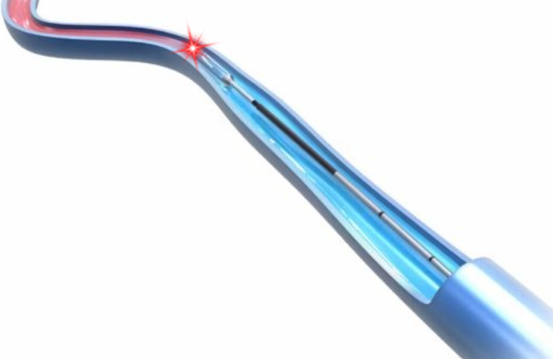
Arfurla Radial Fiber: Precision Endovenous Ablation Probe
This single-patient sterile optical device delivers circumferential laser energy for effective venous closure. Engineered specifically for endovenous laser ablation (EVLA) of varicose veins, its 360° radial emission profile ensures uniform photothermal coagulation of venous walls while mitigating perforation risks and focal overheating.

Length | 3 meters |
Diameter | 400/600um |
Warranty | 6 months |
Application | EVLT, fistula, fissure |
Connector | SMA 905 |
Connection & Sterilization:
Features SMA-905 connector for secure, low-loss laser coupling
Double-barrier ethylene oxide (ETO) sterilization
Individually sealed for guaranteed single-use integrity
Shelf-stable packaging maintains sterility for 3 years


Clinical Advantages:
Consistent Vein Occlusion: Circumferential energy dispersion produces homogeneous thermal denaturation, promoting reliable fibrotic sealing with reduced recanalization rates versus forward-firing fibers.
Enhanced Patient Tolerance: Eliminates forward-directed “hot spots” to minimize postoperative pain, bruising, and perivenous tissue inflammation.
Ultrasound Guidance Optimization: Radiopaque rounded tip enhances echogenicity for precise intraprocedural positioning and controlled pullback under duplex monitoring.
Extended Therapeutic Applications:
Beyond GSV/SSV ablation, suitable for:
Perforator vein thermal ablation
Subdermal coagulation in proctology (hemorrhoids)
Gynecological procedures requiring uniform energy distribution
Critical Handling Protocols:
Sterilization Guidelines:
Approved methods: Ethylene oxide, sodium hypochlorite solution, low-temperature plasma
Strictly avoid autoclaving: High heat causes metal junction degradation
Liquid immersion: Maintain connector above disinfectant level
Optimal exposure: 5-10 minute contact time
Operational Precautions:
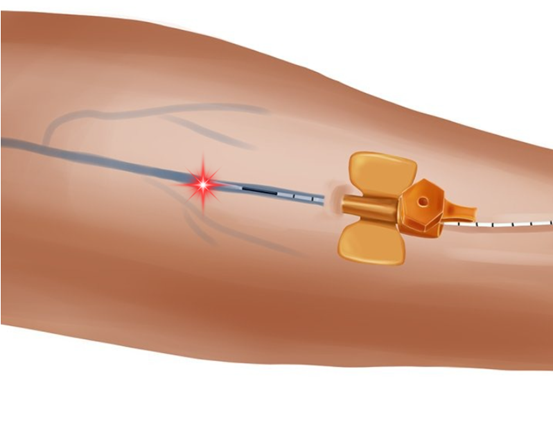
Hemorrhoid treatment requires specialized sharp-tip configuration
During needle insertion: Position only 50% of glass tip beyond cannula (reference instructional imagery)

Safety & Compliance:
Each fiber arrives in validated sterile packaging with traceable lot coding. The radial emission design fundamentally enhances safety profiles during thermal venous procedures by ensuring controlled energy dispersion throughout the treatment zone, establishing this platform as the contemporary standard for endovenous thermoablation therapies.
